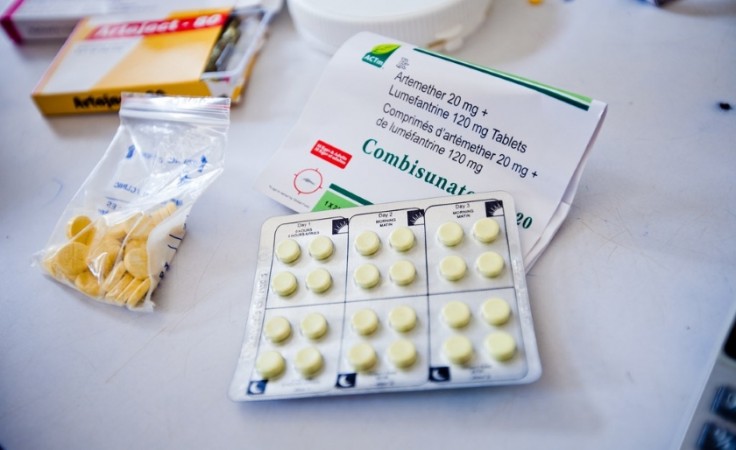
In a groundbreaking achievement for healthcare in Africa, Universal Corporation Limited, a Kenyan pharmaceutical company, has made history by becoming the first manufacturer on the continent to receive approval from the World Health Organization (WHO) for the production of a crucial malaria drug.
The WHO's pre-qualification for the drug, sulfadoxine-pyrimethamine plus amodiaquine (Spaq), is a significant development in the fight against seasonal malaria in children, particularly during peak transmission periods like the rainy seasons. Historically, the demand for drugs like Spaq in Africa has been met through the importation of generic versions from India and China.
Perviz Dhanani, the Managing Director at Universal Corporation Limited, highlighted the importance of this WHO pre-qualification, noting that it marks a pivotal shift towards reducing Africa's dependency on imported medications and strengthening the continent's self-sufficiency in providing essential healthcare solutions.
Africa currently imports over 70% of its drugs, with only six out of hundreds of pharmaceutical companies on the continent achieving WHO "prequalification." Challenges such as high operational costs, limited technical expertise, insufficient investments in the pharmaceutical industry, and concerns about drug regulation and quality have hindered local drug production efforts.
The prevalence of falsified or substandard antimalarials is estimated to contribute to up to 116,000 deaths in sub-Saharan Africa annually. WHO approval, symbolizing compliance with international standards in manufacturing processes and quality control, is expected to facilitate market entry and attract major buyers, including donor-driven organizations.
The local production and equitable distribution of drugs are anticipated to accelerate malaria elimination efforts, according to the anti-malaria research group Medicines for Malaria Venture (MMV). Despite a steady decrease in the number of malaria cases in Africa over the past two decades, concerns have been raised among health experts about the potential reversal of progress due to plateauing funding for the disease.
With a renewed sense of urgency to enhance local production capacity, there is a growing emphasis on a "closer to market" approach in Africa, where more than 95% of global malaria cases and deaths occur. Pierre Hugo, Director of Access at MMV, stressed the importance of ensuring supply security.
This milestone is seen as a significant opportunity for African malaria medicine producers to compete with Indian industry leaders. Joy Phumaphi, Executive Secretary of the African Leaders Malaria Alliance, believes that prioritizing this agenda could benefit millions of children and pregnant women in Africa by providing improved access to malaria protection.
Article by Jed Mwangi
Photo/Google

Comment