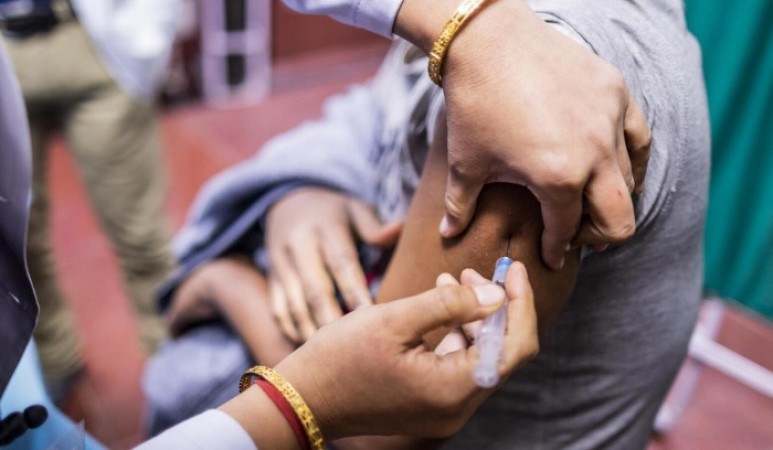
The International AIDS Vaccine Initiative (IAVI) has announced the administration of the first vaccinations in the IAVI G004 Phase 1 HIV vaccine clinical trial, marking a significant milestone in the global pursuit of a safe and effective HIV vaccine.
The first doses were administered on 15 December 2025 at the Perinatal HIV Research Unit (PHRU) in Soweto, South Africa. The experimental regimen represents a novel approach to HIV vaccine development, employing a sequence of three immunogens delivered via an mRNA platform developed in collaboration with Moderna. The trial will assess safety, immune responses, and optimal dose levels, generating critical data to inform subsequent phases of vaccine development.
Notably, the trial is being conducted and led by African investigators, who will also oversee detailed analyses of the immune responses elicited by the vaccine candidates. All participating clinical research sites are based in South Africa, underscoring the country’s central role in cutting-edge HIV research. These sites include the Desmond Tutu Health Foundation (DTHF) Emavundleni and Groote Schuur Hospital Clinical Research Sites (J52) in Cape Town; the eThekwini CAPRISA Research Clinic in Durban; the South African Medical Research Council (SAMRC) Isipingo Clinical Research Centre in Isipingo Rail; the Setshaba Research Centre in Soshanguve; and the Perinatal HIV Research Unit in Soweto.
South Africa, alongside other countries in sub-Saharan Africa, continues to shoulder a disproportionate share of the global HIV burden. Recent estimates indicate that approximately 40.8 million people worldwide were living with HIV in 2024, with more than 1.3 million new infections recorded during the year. These figures underscore the urgent need for innovative prevention tools, including an effective vaccine.
Conducting early-stage vaccine trials in South Africa not only brings research closer to communities most affected by HIV, but also strengthens regional scientific and clinical research capacity. The PHRU, long recognised for its contributions to HIV research, exemplifies the growing leadership of African institutions in complex biomedical studies that were once largely concentrated in North America and Europe.
This trial builds on a strong legacy of HIV vaccine research on the continent, including studies such as the GRAdHIVNE1 T-cell vaccine trial, which involved African principal investigators and multiple research institutions across Zimbabwe and South Africa.
While the initiation of the IAVI G004 trial is a promising development, researchers caution that HIV vaccine development remains a lengthy and challenging endeavour. Previous trials have delivered mixed outcomes, and no licensed HIV vaccine currently exists. However, advances in mRNA technology,accelerated by the success of COVID-19 vaccines, have reinvigorated the field, offering adaptable and powerful tools for next-generation vaccine design.
The IAVI G004 trial will continue over the coming months, with ongoing monitoring of safety and immune responses before progression to larger and more definitive studies. The long-term goal is that vaccines emerging from this research pipeline will contribute meaningfully to reducing new HIV infections and, ultimately, to ending the HIV epidemic.
Photo courtesy / UNICEF
Article by Jed Mwangi

Comment